 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | 'Contrast Enhanced Angiography' | |
Result : Searchterm 'Contrast Enhanced Angiography' found in 0 term [ ] and 2 definitions [ ] and 2 definitions [ ], (+ 17 Boolean[ ], (+ 17 Boolean[ ] results ] results
| previous 6 - 10 (of 19) nextResult Pages :  [1] [1]  [2 3 4] [2 3 4] |  | |  | Searchterm 'Contrast Enhanced Angiography' was also found in the following service: | | | | |
|  |  |
| |
|
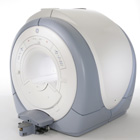
From GE Healthcare;
The Signa HDx MRI system is GE's leading edge whole body magnetic resonance scanner designed to support high resolution, high signal to noise ratio, and short scan times.
Signa HDx 3.0T offers new technologies like ultra-fast image reconstruction through the new XVRE recon engine, advancements in parallel imaging algorithms and the broadest range of premium applications. The HD applications, PROPELLER (high-quality brain imaging extremely resistant to motion artifacts), TRICKS ( contrast- enhanced angiographic vascular lower leg imaging), VIBRANT (for breast MRI), LAVA (high resolution liver imaging with shorter breath holds and better organ coverage) and MR Echo (high-definition cardiac images in real time) offer unique capabilities.
Device Information and Specification CLINICAL APPLICATION Whole body
CONFIGURATION Compact short bore SE, IR, 2D/3D GRE, RF-spoiled GRE, 2DFGRE, 2DFSPGR, 3DFGRE, 3DFSPGR, 3DTOFGRE, 3DFSPGR, 2DFSE, 2DFSE-XL, 2DFSE-IR, T1-FLAIR, SSFSE, EPI, DW-EPI, BRAVO, Angiography: 2D/3D TOF, 2D/3D phase contrast vascular IMAGING MODES Single, multislice, volume study, fast scan, multi slab, cine, localizer H*W*D 240 x 2216,6 x 201,6 cm POWER REQUIREMENTS 480 or 380/415, 3 phase ||
COOLING SYSTEM TYPE Closed-loop water-cooled grad. | |  | | | |
|  | |  |  |  |
| |
|

From Philips Medical Systems;
the Intera-family offers with this member a wide range of possibilities, efficiency and a ergonomic and intuitive serving-platform. Also available as Intera CV for cardiac and Intera I/T for interventional MR procedures.
The scanners are also equipped with SENSE technology, which is essential for high-quality contrast enhanced magnetic resonance angiography, interactive cardiac MR and diffusion tensor imaging ( DTI) fiber tracking.
The increased accuracy and clarity of MR scans obtained with this technology allow for faster and more accurate diagnosis of potential problems like patient friendliness and expands the breadth of applications including cardiology, oncology and interventional MR.
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
Standard: head, body, C1, C3; Optional: Small joint, flex-E, flex-R, endocavitary (L and S), dual TMJ, knee, neck, T/L spine, breast; Optional phased array: Spine, pediatric, 3rd party connector; Optional SENSE coils: Flex-S-M-L, flex body, flex cardiac
SE, Modified-SE ( TSE), IR (T1, T2, PD), STIR, FLAIR, SPIR, FFE, T1-FFE, T2-FFE, Balanced FFE, TFE, Balanced TFE, Dynamic, Keyhole, 3D, Multi Chunk 3D, Multi Stack 3D, K Space Shutter, MTC, TSE, Dual IR, DRIVE, EPI, Cine, 2DMSS, DAVE, Mixed Mode; Angiography: PCA, MCA, Inflow MRA, CE
TR
2.9 (Omni), 1.6 (Power), 1.6 (Master/Expl) msec
TE
1.0 (Omni), 0.7 (Power), 0.5 (Master/Expl) msec
RapidView Recon. greater than 500 @ 256 Matrix
0.1 mm(Omni), 0.05 mm (Pwr/Mstr/Expl)
128 x 128, 256 x 256,512 x 512,1024 x 1024 (64 for BOLD img.)
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
| |  | |
• View the DATABASE results for 'Intera 1.5T™' (2).
| | | | |
|  | |  |  |  |
| |
|

From Siemens Medical Systems;
The MAGNETOM 7T is designed as an open research platform. 7T MRI provides anatomical detail at the submillimiter scale, enhanced contrast mechanisms, outstanding spectroscopy performance, ultra-high resolution functional imaging ( fMRI), multinuclear whole-body MRI and functional information.
This ultra high field (UHF) MRI device is a research system and not cleared, approved or licensed in any jurisdiction for patient examinations.
Device Information and Specification
CLINICAL APPLICATION
Whole body
High-performance, ultra high field coils available. Integration and support for coil developments.
CHANNELS (min. / max. configuration)
32, optional 8 channels TX array
40 x 40 x 30 cm³ less than 8% nonlinearity
MAGNET WEIGHT (gantry included)
35017 kg
DIMENSION H*W*D (gantry included)
320 x 240 x 317,5 cm
MAX. AMPLITUDE
up to 70 mT/m
Up to 3rd order shim coils, user configurable B0 shim ? B0 maps and ROI definition
POWER REQUIREMENTS
2000 Volts, 650A
| |  | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Contrast Enhanced Angiography' was also found in the following service: | | | | |
|  |  |
| |
|
( MRA) Magnetic resonance angiography is a medical imaging technique to visualize blood filled structures, including arteries, veins and the heart chambers. This MRI technique creates soft tissue contrast between blood vessels and surrounding tissues primarily created by flow, rather than displaying the vessel lumen. There are bright blood and black blood MRA techniques, named according to the appearance of the blood vessels. With this different MRA techniques both, the blood flow and the condition of the blood vessel walls can be seen. Flow effects in MRI can produce a range of artifacts. MRA takes advantage of these artifacts to create predictable image contrast due to the nature of flow.
Technical parameters of the MRA sequence greatly affect the sensitivity of the images to flow with different velocities or directions, turbulent flow and vessel size.
This are the three main types of MRA:
All angiographic techniques differentially enhance vascular MR signal. The names of the bright blood techniques TOF and PCA reflect the physical properties of flowing blood that were exploited to make the vessels appear bright. Contrast enhanced magnetic resonance angiography creates the angiographic effect by using an intravenously administered MR contrast agent to selectively shorten the T1 of blood and thereby cause the vessels to appear bright on T1 weighted images.
MRA images optimally display areas of constant blood flow-velocity, but there are many situations where the flow within a voxel has non-uniform speed or direction. In a diseased vessel these patterns are even more complex. Similar loss of streamline flow occurs at all vessel junctions and stenoses, and in regions of mural thrombosis. It results in a loss of signal, due to the loss of phase coherence between spins in the voxel.
This signal loss, usually only noticeable distal to a stenosis, used to be an obvious characteristic of MRA images. It is minimized by using small voxels and the shortest possible TE. Signal loss from disorganized flow is most noticeable in TOF imaging but also affects the PCA images.
Indications to perform a magnetic resonance angiography ( MRA):
•
Detection of aneurysms and dissections
•
Evaluation of the vessel anatomy, including variants
•
Blockage by a blood clot or stenosis of the blood vessel caused by plaques (the buildup of fat and calcium deposits)
Conventional angiography or computerized tomography angiography (CT angiography) may be needed after MRA if a problem (such as an aneurysm) is present or if surgery is being considered.
See also Magnetic Resonance Imaging MRI. | | | | | | | | | | |
• View the DATABASE results for 'Magnetic Resonance Angiography MRA' (3).
| | |
• View the NEWS results for 'Magnetic Resonance Angiography MRA' (10).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
ABLAVAR™ (formerly named Vasovist™) is a blood pool agent for magnetic resonance angiography ( MRA), which opens new medical imaging possibilities in the evaluation of aortoiliac occlusive disease (AIOD) in patients with suspected peripheral vascular disease.
ABLAVAR™ binds reversibly to blood albumin, providing imaging with high spatial resolution up to 1 hour after injection, due to its high relaxivity and to the long lasting increased signal intensity of blood.
As with other contrast media: the possibility of serious or life-threatening anaphylactic or anaphylactoid reactions, including cardiovascular, respiratory and/or cutaneous manifestations, should always be considered.
WARNING:
NEPHROGENIC SYSTEMIC FIBROSIS
Gadolinium-based contrast agents increase the risk for nephrogenic systemic fibrosis (NSF) in patients with acute or chronic severe renal insufficiency (glomerular filtration rate less than 30 mL/min/1.73m 2), or acute renal insufficiency of any severity due to the hepato-renal syndrome or in the perioperative liver transplantation period.
See also Cardiovascular Imaging, Adverse Reaction, Molecular Imaging, and MRI Safety.
Drug Information and Specification
NAME OF COMPOUND
Diphenylcyclohexyl phosphodiester-Gd-DTPA, gadofosveset trisodium, MS-325
T1, predominantly positive enhancement
20-45 mmol-1sec-1, Bo=0,47T
PHARMACOKINETIC
Intravascular
CONCENTRATION
244 mg/mL, 0.25mmol/mL
DOSAGE
0.12 mL/kg, 0.03 mmol/kg
DEVELOPMENT STAGE
FDA approved
DO NOT RELY ON THE INFORMATION PROVIDED HERE, THEY ARE
NOT A SUBSTITUTE FOR THE ACCOMPANYING
PACKAGE INSERT!
Distribution Information
TERRITORY
TRADE NAME
DEVELOPMENT
STAGE
DISTRIBUTOR
USA, Canada, Australia
ABLAVAR™
Approved
| |  | |
• View the DATABASE results for 'ABLAVAR™' (3).
| | |
• View the NEWS results for 'ABLAVAR™' (1).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
News & More:
| |
| |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |